Update: Per the original recall detailed below, many Phillips DreamStations were reworked in order to properly function. Now these reworked DreamStations are being recalled once again, and it’s a Class I recall – the most serious type of recall. Use of these devices may cause serious injuries, serious health consequences, or even death.
The latest issue is that some devices were assigned incorrect or duplicate serial numbers during initial programming. This duplication can cause therapy to be delivered using the wrong prescription or the factory default settings. Additionally, it may fail to deliver any therapy at all. Unfortunately, there is no warning or indication to the user that the DreamStation is not working the way the doctor intended or prescribed.
Original recall details:
Phillips Respironics is recalling its ventilators, BiPAP machines, and CPAP machines due to potential health risks. The issue lies within the polyester-based polyurethane sound abatement foam. It’s meant to reduce sounds and vibrations in these devices, which allow users to sleep in relative peace while wearing the machine.

However, the foam has the potential to break down and enter the device’s air pathway. If this occurs, black debris from the foam or certain chemicals released into the device’s air pathway may be inhaled or swallowed by the person using the device.
The affected devices were manufactured between 2009 and April 26, 2021. Below is the full list of recalled devices. If you use one of these affected devices, please talk to your health care provider to decide on a suitable treatment for your condition. And below this chart is a list of recommendations for people who use these recalled machines.
Recalled devices:
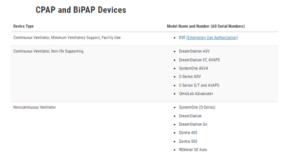
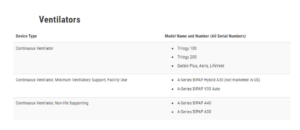
BiPap or CPAP: Recommendations for users and their caregivers
Talk to your health care provider to decide on a suitable treatment for your condition, which may include:
- Stopping use of your device
- Using a similar device that is not part of the recall
- Using alternative treatments for sleep apnea, such as positional therapy or oral appliances, which are similar to a sports mouthguard or an orthodontic retainer
- Initiating long-term therapies for sleep apnea, such as losing weight, avoiding alcohol, stopping smoking, or surgical options
- Continuing to use your affected device, if your health care provider determines that the benefits outweigh the risks identified in the recall notification
Follow the manufacturer’s instructions and recommended cleaning and replacement guidelines for your CPAP machine and accessories. Ozone cleaners may worsen the breakdown of the foam, and there are other potential risks associated with the use of ozone and ultraviolet (UV) light products for cleaning CPAP machines and accessories.
You can also register your device(s) on Philips Respironics’ recall website to stay informed of updates, such as new instructions or other corrective fixes, which the FDA is requiring. Please report any problems with a device through the FDA’s MedWatch Voluntary Reporting Form.
Ventilators: Recommendations for caregivers or people using affected ventilators
- Do not stop or change ventilator use until you have spoken with your health care provider.
- Alternate ventilator options for therapy may not exist or may be severely limited for patients who require a ventilator for life-sustaining therapy, or in cases where therapy disruption is unacceptable.
- Talk to your health care provider about using an inline bacterial filter, which may help to filter out particles of foam, as indicated in the Philips recall notification.
At this time, the FDA does not have evidence of the safety and effectiveness of a filter for mitigating the foam risks, and the FDA’s evaluation is ongoing. It is important to note the following considerations:
- Filters will not help to reduce exposure to certain chemicals that may be released from the foam.
- Filters may affect ventilator performance because they may increase the resistance of air flow through the device.
- You should closely monitor for possible accumulation of foam debris on the filter or resistance-related problems in the breathing circuit after filter placement.
- Register your device(s) on Philips Respironics’ recall website.
- Report any problems with a device through the FDA’s MedWatch Voluntary Reporting Form




